Nutritional Deficiency in Roses
by Ted Burg
This article is a 2017 ARS AOM winner
When your roses are looking stressed, have yellow foliage with green veins or leaf edges are brown, it’s time to look at nutrient deficiencies in your roses and what may be causing it. Nutrient availability is highly affected by soil acidity. The acidity of the soil is measured by the pH on a logarithmic scale running from 0 to 14. A pH of 0 is very highly acidic (think battery acid) and a pH of 14 (think lye) is very highly alkaline (basic). A pH of 7.0 is considered neutral.
A soil may have sufficient levels of nutrients, but if the soil pH is very far from the ideal pH for roses (6.0 -6.5), the nutrients may not be available to the plant. This is because in certain pH ranges, nutrients will react with each other and form insoluble compounds. This is frequently referred to as the nutrients being "locked up."
In some cases, this can be overcome by increasing the amount of the nutrient added to the soil. However, adjusting the soil pH is typically more effective. The width of bars in the graph to the right, indicates the availability of the nutrient to plants.
Most nutrients become available in a pH range of 6.0-6.5.
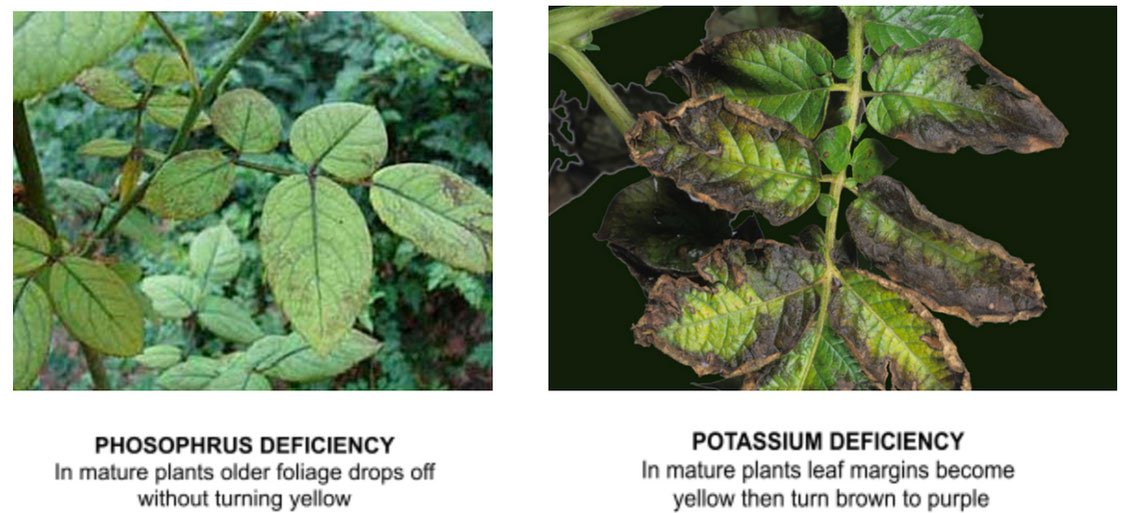
Note that phosphorus is available between a pH of 6.0-7.0. Below 6.0, phosphorus reacts with iron to form an insoluble iron phosphate. Above a pH of 7.0, phosphorus reacts with calcium to form insoluble calcium phosphates.
Iron and manganese both decline in availability about 100 times for each 1.0 increase in pH. They become seriously deficient in alkaline (high pH) soils. Aluminum is not needed by plants, but can cause serious toxicity problems if the pH gets below 5.5. When the pH gets below 5.5, aluminum leaves the crystal structure of silicate clays and interferes with root growth. Soils that are high in organic matter are less affected by lower pH.
Most cases of nutrient deficiency are caused by some interference in the availability or uptake of these elements from the soil, rather than by an actual shortage of these nutrients in the soil. Poor aeration, over watering, high levels of soluble salts, and high or low pH may cause the nutrients to be unavailable to the plant.
It is important to note that many symptoms of a nutrient deficiency are problems with availability rather than supply. The nutrients may be present in the soil but are unavailable because of a pH that is too high or too low. There may also be a nutrient imbalance that prevents absorption of one nutrient thus causing symptoms of deficiency. Many symptoms are similar. Generally, micronutrient deficiencies are rarely seen. The most common deficiencies are nitrogen, iron, oxygen, plus heat stress.